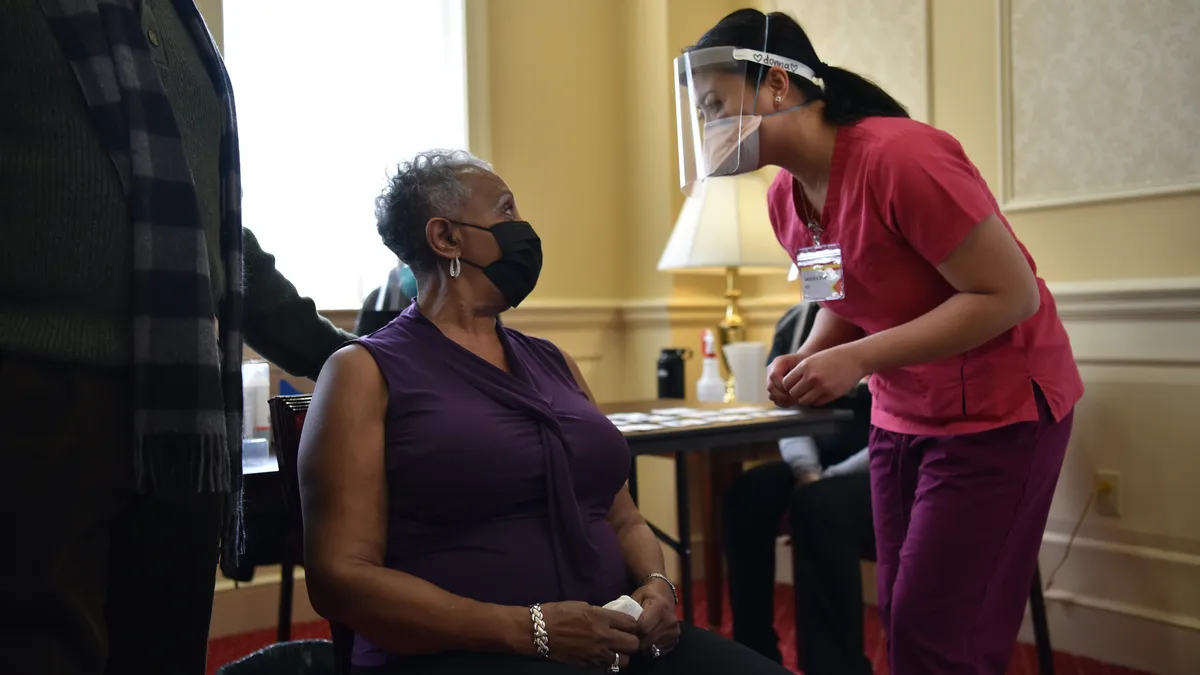Government: Page 71
-
CMS pushes back kidney care payment model start date to January 2022
It is the second Trump-era payment model President Joe Biden's health administration has recently tweaked.
By Rebecca Pifer Parduhn • March 10, 2021 -
HHS will send $250M in grants for vaccine equity push
The funding is available to cities, counties and other subdivisions and is expected to produce 30 urban projects and 42 rural projects over the next two years.
By Shannon Muchmore • March 9, 2021 -
FDA grants first EUA to at-home OTC molecular test for COVID-19
Cue Health received the emergency authorization and expects to be able to produce more than 100,000 tests a day by the summer, as the agency continues to prioritize more at-home testing options.
By Nick Paul Taylor • March 8, 2021 -
Coronavirus relief bill with rural hospital aid passes Senate
The nearly $2 trillion bill also includes billions for COVID-19 testing and contact tracing as well as extra subsidies to help people buy health coverage through the Affordable Care Act marketplaces.
By Shannon Muchmore • March 8, 2021 -

 "Medical disposable masks on wooden background" by Marco Verch Professional Photographer and Speaker is licensed under CC BY 2.0
"Medical disposable masks on wooden background" by Marco Verch Professional Photographer and Speaker is licensed under CC BY 2.0
Becerra tells Californians to dispute COVID-19 fees from providers
Patients charged a "COVID fee" from a recent visit should contact their insurer and request a reimbursement, according to a statement from the state's attorney general, who is the nominee for HHS secretary.
By Hailey Mensik • March 4, 2021 -
Georgia health systems discard merger plans, averting FTC challenge
The tie-up between two of the largest systems in central Georgia would have led to "significant harm" for area residents and businesses in the form of higher healthcare costs, the agency alleged.
By Samantha Liss • March 4, 2021 -
FDA lays out data modernization action plan
"Even small advances in our ability to gain useful insights from data can represent significant opportunities," agency officials wrote in a blog post.
By Nick Paul Taylor • March 4, 2021 -
Merck to help J&J make vaccine doses in White House-brokered deal
Merck will lend J&J the use of two separate facilities in the unusual agreement, which was brokered by the Biden administration and could help double supply of the one-dose shot.
By Jonathan Gardner • Updated March 2, 2021 -

 Retrieved from C-SPAN on February 24, 2021
Retrieved from C-SPAN on February 24, 2021
Becerra nomination heads to full Senate vote
The 51-48 vote Thursday means arguments and a full floor vote on the nominee will now be scheduled. The only Republican to approve of advancing Becerra was Sen. Susan Collins of Maine.
By Shannon Muchmore • Updated March 11, 2021 -
One Medical CEO says 'we are not perfect,' vows to improve amid Congress probe
"We remain committed to taking a hard look at our efforts and finding ways to continuously improve," Amir Dan Rubin said amid reports the primary care chain gave the coronavirus vaccine to ineligible people.
By Rebecca Pifer Parduhn • Updated March 5, 2021 -
FDA authorizes J&J's 1-shot coronavirus vaccine
The agency's emergency clearance makes J&J's vaccine the third available in the U.S., adding much-needed reinforcements at a critical time.
By Ned Pagliarulo • Updated March 1, 2021 -
Deep Dive
4 healthcare antitrust issues to watch
The FTC is looking to get more aggressive with anticompetitive tie-ups while states eye ways to beef up oversight. And if handed the reins of HHS, Xavier Becerra would likely put an antitrust lens to potential rules.
By Samantha Liss • March 1, 2021 -
Former Senate aide Elizabeth Fowler to lead CMS innovation center
As head of the Center for Medicare and Medicaid Innovation, the former chief health counsel to Democrats will oversee a $10 billion fund to test new payment models.
By Rebecca Pifer Parduhn • March 1, 2021 -
FTC abandons challenge to Jefferson Health-Einstein merger, allowing deal to proceed
The union between the two Philadelphia providers, first announced two years ago, is now expected to close within six months.
By Samantha Liss • Updated March 1, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
CMS moves to stop COVID-19 testing denials, cost sharing in private plans
Congress required comprehensive health plans to cover COVID-19 tests without cost sharing, prior authorization or medical management last year, only for guidance to create uncertainty about the rules.
By Nick Paul Taylor • March 1, 2021 -
Hospitals likely upcoding severity levels for Medicare patients, OIG says
The most expensive hospital stays increased 20% over the six years studied, which were before the COVID-19 pandemic began, according to the report.
By Ron Shinkman • Feb. 26, 2021 -
FDA panel backs J&J's coronavirus vaccine, clearing way for shot's authorization
In a unanimous vote, the panel of experts gave a green light to the FDA for clearing J&J's shot, judging the benefits of vaccination outweigh its risk.
By Ned Pagliarulo • Feb. 26, 2021 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

FDA advisers meet to review J&J's one-shot coronavirus vaccine
Friday's advisory committee meeting is one of the last steps in the FDA's review and could clear the way for an emergency authorization within days.
By Ben Fidler , Ned Pagliarulo , Jonathan Gardner • Updated Feb. 26, 2021 -

 Retrieved from C-SPAN on February 24, 2021
Retrieved from C-SPAN on February 24, 2021
Becerra distances himself from 'Medicare for All,' appears on track for confirmation
The nominee to run HHS also voiced support for expanded reimbursement of virtual care beyond the COVID-19 pandemic. "I don't think we're going back to the old days when it comes to telehealth," he said Wednesday.
By Shannon Muchmore • Feb. 25, 2021 -

 Retrieved from C-SPAN on February 23, 2021
Retrieved from C-SPAN on February 23, 2021
Becerra backs price transparency, provider competition at first Senate panel
President Joe Biden's pick for HHS chief turns Wednesday to the finance panel, which will vote on sending the nomination to the full Senate.
By Shannon Muchmore • Feb. 24, 2021 -
FDA review supports safety, efficacy of J&J coronavirus vaccine
Agency scientists noted the shot's strong protection against severe COVID-19, even for the virus variant first detected in South Africa and known to weaken vaccine potency.
By Ned Pagliarulo , Jonathan Gardner • Feb. 24, 2021 -
FDA lays out shortened path for testing vaccines against new coronavirus variants
Lengthy trials won't be needed for updating shots against emerging viral strains, the agency told developers in newly published guidance.
By Ned Pagliarulo • Feb. 23, 2021 -

 Retrieved from C-SPAN on February 23, 2021
Retrieved from C-SPAN on February 23, 2021
Becerra embraces healthcare antitrust record at first Senate hearing
Sen. Richard Burr, R-N.C., gave a taste of GOP opposition to the nominee for HHS Secretary on Tuesday by accusing him of disregarding the value of private sector innovation.
By Shannon Muchmore • Feb. 23, 2021 -
FDA starts review of how skin pigmentation affects pulse oximeter results
The agency is evaluating published literature related to factors that may affect device accuracy and performance following pressure from senators to address concerns that "racism may be embedded in key clinical tools."
By Nick Paul Taylor • Feb. 22, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
Why this week's FDA meeting on J&J's coronavirus vaccine will be important
The FDA is widely expected to authorize the drugmaker's one-dose shot. But the advisory committee meeting beforehand will offer a window into debate over several key issues.
By Ben Fidler , Ned Pagliarulo • Feb. 22, 2021