Government: Page 69
-
Survey casts doubt on utility of wearable devices in healthcare
Forrester's report, based on interviews with doctors, patients and vendors, suggests data gaps and physician skepticism of the popular products is too high for widespread adoption, although design changes could remedy that.
By Ron Shinkman • April 22, 2021 -
ACA navigator funds get massive boost to record level
The agency also said about 12 million people enrolled in an Affordable Care Act plan during the 2021 open enrollment period, a 5% increase from the year prior.
By Shannon Muchmore • April 22, 2021 -
Value-based care at 'critical juncture,' new CMMI chief says
Even though some testing payment models have been delayed or discarded, CMMI's new leader said the agency remains committed to finding models that reward value not volume.
By Rebecca Pifer Parduhn • April 20, 2021 -
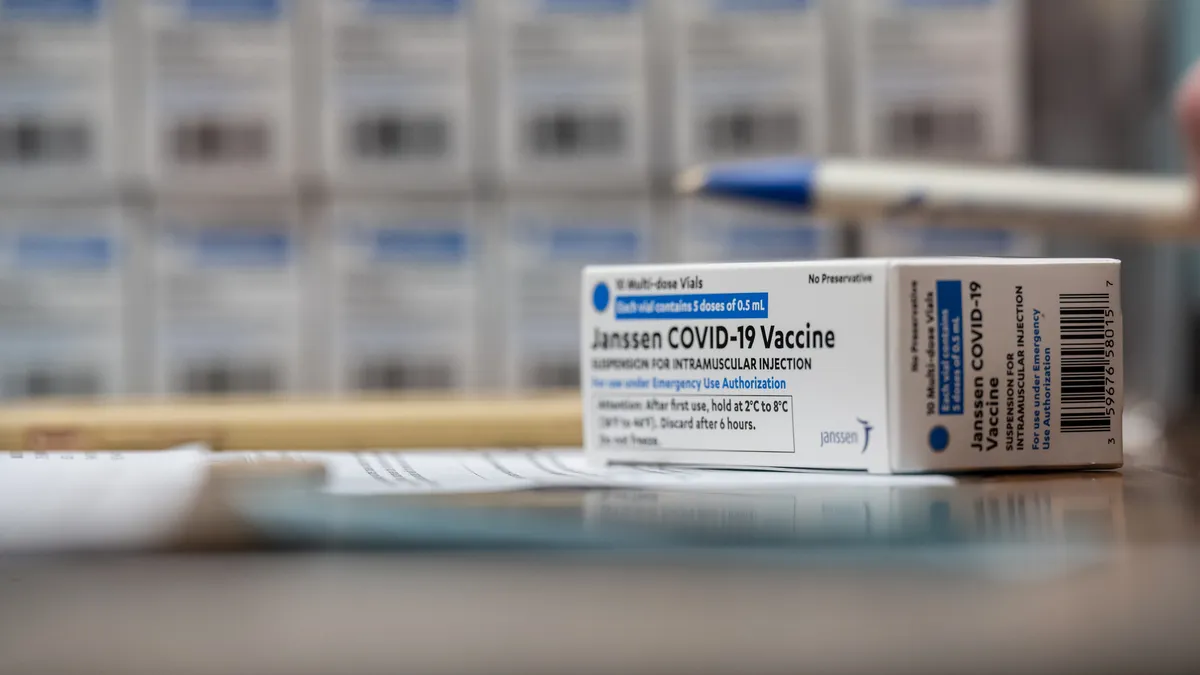
J&J to resume vaccine rollout in Europe after regulator says benefits outweigh risks
The EMA, however, recommended a warning be added to highlight a rare but serious side effect. Regulators in the U.S. are expected to soon make a similar decision.
By Ben Fidler , Jonathan Gardner • Updated April 20, 2021 -
Humana nets nearly $200M in overpayments, OIG audit finds
The watchdog said the payer submitted documentation that inaccurately showed some of its Medicare Advantage members were sicker than they actually were.
By Samantha Liss • April 20, 2021 -
Healthcare employment steadily rebounding though hospitals left out
Hospital employment has sunk for three consecutive months, according to data from the Altarum Institute, and is down 37,000 jobs since the end of last year.
By Ron Shinkman • April 19, 2021 -
Advocate Aurora Health's new investment unit is on the hunt for deals
The Midwestern giant joins other nonprofits in launching a for-profit unit to diversify its revenue streams. Some critics question whether the strategy fits with the mission and perks enjoyed by not-for-profit entities.
By Samantha Liss • April 19, 2021 -
HHS nominees get smooth confirmation hearing
The scene was a contrast to the sometimes heated questioning of HHS Secretary Xavier Becerra, who was eventually confirmed by a narrow margin.
By Shannon Muchmore • April 16, 2021 -
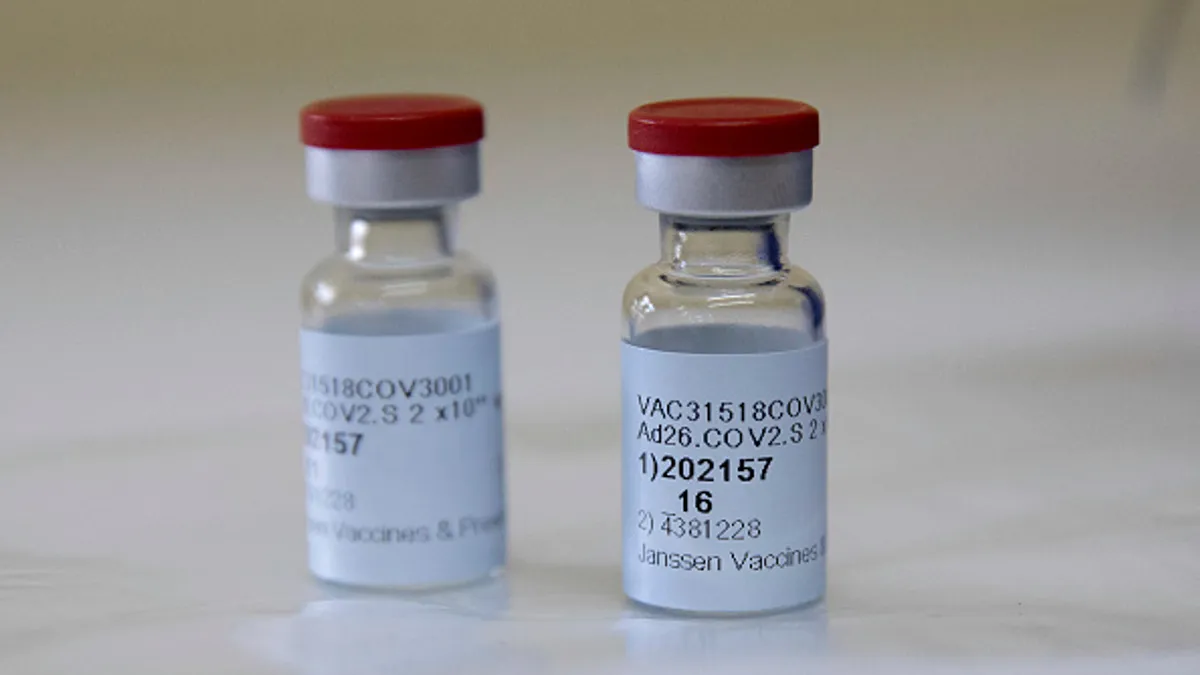
The US paused use of J&J's vaccine. What happens next?
A call by regulators to stop J&J vaccinations won't dramatically disrupt supply in the U.S. But changes in labeling are possible, as is a renewed debate over vaccine hesitancy.
By Ned Pagliarulo , Ben Fidler , Jonathan Gardner • April 14, 2021 -
CDC panel delays decision on J&J vaccine, extending pause over rare side effect
Advisers to the agency agreed to wait for more data before recommending new guidance, but aim to reconvene quickly to decide whether J&J vaccinations should be resumed, and for whom.
By Jonathan Gardner • April 14, 2021 -

 Retrieved from Official White House Photo by Adam Schultz.
Retrieved from Official White House Photo by Adam Schultz.
Medicare sequester cut pause extended through 2021
The 2% cuts were first put on hold more than a year ago through federal coronavirus relief legislation. To pay for the extension, the new bill states the cuts will increase in 2030.
By Hailey Mensik • April 14, 2021 -
Lawmakers urge HHS to conduct 'vigorous oversight' of hospital price posts after reports of noncompliance
Hospitals are now required to publicly post the prices they negotiate with insurers online, but noncompliance is common. A bipartisan group from the House voiced their discontent in a letter to HHS Secretary Xavier Becerra.
By Samantha Liss • April 14, 2021 -
Surprise Billing
Patients hit with surprise medical bills paid ER docs 10 times more than others
The findings published in Health Affairs suggest why some ER groups resist coming in-network with insurers: They stand to collect more in revenue if they stay outside a network, posing a financial risk to patients.
By Samantha Liss • April 13, 2021 -
US calls for pause in J&J coronavirus vaccine rollout after rare blood clotting cases
The FDA and CDC recommended halting vaccinations while they investigate six cases of unusual blood clots in women given the company's shot.
By Ben Fidler , Ned Pagliarulo • Updated April 13, 2021 -
Researchers lay out hypothesis for rare side effect linked to AstraZeneca vaccine
EU regulators have reiterated the benefits of the shot outweigh the risks, but the safety concerns are another hurdle to the vaccine's rollout in Europe.
By Ned Pagliarulo , Ben Fidler • April 9, 2021 -
Medtech industry, surgeons push Medicare to pull back prior authorization rules
The medtech lobby joined 39 stakeholders in warning CMS that including cervical fusion with disc removal and implanted spinal neurostimulators as new service categories will delay patient access to medically necessary procedures.
By Greg Slabodkin • April 8, 2021 -
AstraZeneca's coronavirus vaccine possibly linked to rare blood clots, EMA says
Europe's drug regulator affirmed the benefits of vaccination outweigh the risks, but recommended the shot's label be updated to warn of the newly established side effect.
By Ben Fidler • Updated April 7, 2021 -
Urgent care centers draw some ER visits but associated with higher spending overall
The findings published in Health Affairs may suggest patients are going to the centers instead of more appropriate and lower cost settings like a primary care office or virtual visit.
By Shannon Muchmore • April 7, 2021 -
Nonprofit hospitals spent less on charity care than for-profit, government facilities: Health Affairs
The research is likely to garner attention as skeptics have questioned whether nonprofit organizations dole out enough charity care to justify the tax breaks they receive.
By Samantha Liss • April 6, 2021 -
Black patients far more likely to experience discrimination when seeking care: study
Reports of discrimination based on race, ethnicity, disability, gender, sexual orientation or health condition were even higher for low-income Black adults and Black women, according to a new study by the Urban Institute.
By Ron Shinkman • April 5, 2021 -
FDA warns of patient deaths tied to reusable urological endoscopes
The agency is sounding the alarm after receiving more than 450 adverse event reports in four years tying patient infections to the devices, as it continues to track contamination issues in duodenoscopes.
By Susan Kelly • Updated April 5, 2021 -
Shifting payer mix puts 340B hospitals at risk of losing eligibility
AHA wants HHS to waive certain eligibility requirements to allow continued access to the program during the public health emergency, the group wrote in a letter to Secretary Xavier Becerra.
By Hailey Mensik • April 1, 2021 -
A year into the pandemic, advanced cancer diagnoses are rising
Research from radiation oncologists and molecular pathologists add to evidence showing that many people skipped getting cancer screenings in 2020 as they avoided going to the doctor to minimize the risk of COVID-19 infection.
By Susan Kelly • March 31, 2021 -
FTC challenges Illumina-Grail deal, putting $7B merger in jeopardy
The commission said the union will "harm competition in the U.S. market for life-saving multi-cancer early detection tests." Illumina is opposing the action, but Wall Street analysts see no “easy fixes."
By Nick Paul Taylor • March 31, 2021 -

 The image by Original image from Carol M. Highsmith’s America, Library of Congress collection is licensed under CC BY 2.0
The image by Original image from Carol M. Highsmith’s America, Library of Congress collection is licensed under CC BY 2.0
Medical liability insurance premiums rising after stable decade, AMA report finds
While providers grapple with the pandemic's toll on finances, some can face liability premiums of $200,000 a year, according to the analysis sponsored by the doctors' lobby.
By Hailey Mensik • March 30, 2021